Case Study Series
Retrospective Patient Follow Up–HiveTM PL & C Interbody Systems
Dwight Tyndall, MD
Lakeshore Bone and Joint Institute
Munster, IN
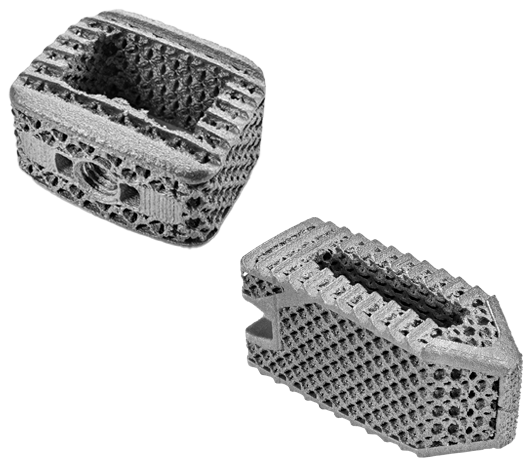
Technology Background
The use of interbody fusion cages, including cervical and lumbar fusion cages, in the treatment of spondylolisthesis and spinal deformity is common and understanding factors affecting successful fusion is critical to improving outcomes.
One of the most commonly employed techniques for achieving fusion is implantation of an interbody cage. A study from the Nationwide Inpatient Sample database showed as many as 83% of surgeries for degenerative spondylolisthesis involve the use of an interbody cage [1]. Titanium was first used in interbody cages because it enhanced cell adhesion and osseointegration favoring bone fusion [2]. However, it may have had a higher rate of subsidence compared to polyetheretherketone (PEEK) due to differences in the modulus of elasticity [3,4]. Despite that, PEEK is chemically inert with limited cell adhesion and fixation to bone [5].
Advocates for PEEK devices have recognized the need for reduced stiffness, as compared to subtractively manufactured titanium implants; however, PEEK lacks the beneficial cellular characteristics of titanium. Titanium surfaces where manufacturers can control tissue interaction to the nano-scale propose to offer even greater advantages.
Frequently, titanium 3D printed implants rely on rigid framed structures, shells, and solid noses and tails to bear the loads required for rigorous mechanical performance demands. The result increases construct stiffness and reduces load on internal bone graft.
Alternate approaches to internal porosity utilize randomly generated structures prone to unknown failure points, inconsistent or reduced bone loading, and to choke points for fluid flow. Truss structures are prone to loss of graft material, particularly during insertion, and do not provide substantial surface area to support tissue during healing.
The design goals for the Hive™ implant, with Soft Titanium® technology, were to offer a powerful feature set, including:
- a supporting scaffold for bone growth
- ideal pore characteristics for ingrowth
- maximal surface for bone to attach
- post-operative visibility
- mechanical compatibility
As a result, the Hive scaffold has an overall stiffness comparable to PEEK. Mechanical performance testing for spine implants is conducted in accordance with ASTM F2077 for evaluation of the axial strength and stiffness of the tested device modulus.
Soft Titanium, with modulus of elasticity and stiffness that is matched closely to normal bone, has two potential advantages for obtaining solid fusion after spine surgery. One advantage is the potential benefit of allowing some elastic deformation during regular activities, which in turn will place more loading on the healing fusion without destabilizing the construct and may influence improved bone formation at the fusion site. The second potential advantage of a lower stiffness cage is lower risk of subsidence, especially in osteoporotic patients, which may prevent compromised fixation and loss of correction. This loss of correction can impact alignment and indirect decompression, subsequently potentially negatively affecting clinical outcomes. A cage with lower stiffness is also potentially less likely to violate the endplates on insertion, which can also prevent subsidence.
The purpose of this study is to report on one surgeon’s experience with the Soft Titanium technology in cervical and posterior lumbar cages.
Methods
Twenty-seven patients underwent instrumented interbody fusion from September 2021 to April 2022 with Hive interbody devices. Fifteen patients were male and 12 were female. The average age was 53 yo, and average BMI was 31. The average pre-op Visual Analog Scale (VAS) Pain Score was 7.3/10.
Twenty-two cases were lumbar posterior interbody fusions (TLIF or PLIF) and five were anterior cervical discectomy and fusions (ACDF).
The posterior lumbar fusion cases were performed between L3 and S1 levels, with one Hive PL (straight) interbody cage placed obliquely across the disc space for a TLIF, or two Hive PL cages placed in parallel, for a PLIF. Local autologous bone was packed in the cage as well as around the cage, in the interbody space. Additional bone graft was placed bilaterally in the posterolateral gutters. Fusion levels were backed up with pedicle screws. Indications for fusion were lumbar degenerative spondylolisthesis with stenosis and lumbar degenerative disc disease.
The anterior cervical cases were performed as single level ACDF’s, between the C2 and C6 vertebral bodies, with Hive C Interbody Devices, and were all supplemented with anterior cervical plates. Allograft bone graft was packed into each cage. Indications for fusion were cervical stenosis and cervical disc herniation with radicular symptoms.
Patients were followed for approximately six months and discharged once their preoperative pain was reduced and the index level was assessed as fused and stable.
Results
- 27 patients
- Average last follow-up 7-months (5 – 13 months)
- Avg pain reduction – 4.8 / 10
- 26/27 (96.3%) cervical and lumbar fusion patients are reported as fused or continuing towards bony consolidation across the disc space, with stable constructs and no evidence of subsidence
Conclusions
Hive interbody cages, for both the cervical and lumbar fusions, have unique characteristics which makes them ideally suited for performing interbody fusions, specifically:
- The lattice of the cages are such that the cages serve as a reservoir for blood and bone marrow to aid in the fusion. As bone graft is packed in the cage, the blood/bone marrow that accompanies this bone graft is able to be “absorbed” by the cage leading to the entire cage to be filled with bio-mass.
- The lattice of the cages allows for decreased titanium density, leading to a transparency effect on radiographs and less scatter on post-op CT and MRI scans.
- The NanoHive cages are engineered with lower stiffness to reduce the chance of subsidence and, consequently, decrease the risk of pseudarthrosis.
- These characteristics ultimately lead to reduced failures, ie pseudarthrosis, and therefore improved fusion rates and clinical results.
Patient #1
A 42 year old male, with a BMI of 33.2, presented with a large recurrent disc herniation and collapse of the L4-L5 disc space
—
An instrumented lumbar fusion (TLIF) with an interbody at L4-L5 was recommended
—
At the 3 month follow up, the construct was stable and there was no collapse or subsidence noted. The interbody fusion appeared to be consolidating well
—
At the 6 month follow up, plain radiographs showed an instrumented fusion at L4-5. The patient’s VAS Pain Score had decreased from 8/10 pre-op, to 0/10
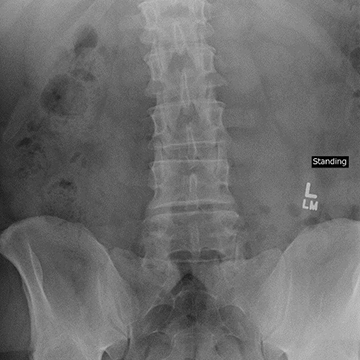
Pre-Op
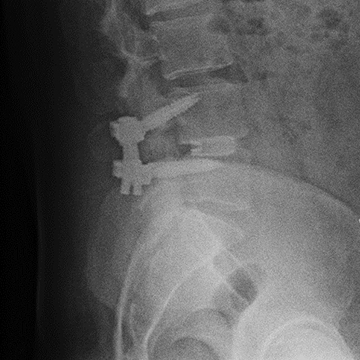
6 Months
Patient #2
A 72 year old female, with a BMI of 25, presented with degenerative spondylolisthesis, with severe central and lateral stenosis at L4-L5 and an L5-S1 collapse
—
An instrumented lumbar fusion (TLIF) with an interbody at L4-L5 was recommended
—
At the 3 month follow up, there was no collapse or subsidence noted, as well as no evidence of looseness of instrumentation
—
At the 6 month follow up, there was no collapse or subsidence noted. A CT scan showed a solidifying lumbar fusion, and the posterior lateral fusion along left side was also solidified. The patient’s VAS Pain Score had decreased from 6/10 pre-op to 0/10
—
At 9 months, plain radiographs showed an instrumented fusion at L4-5. The instrumentation and the construct appear stable
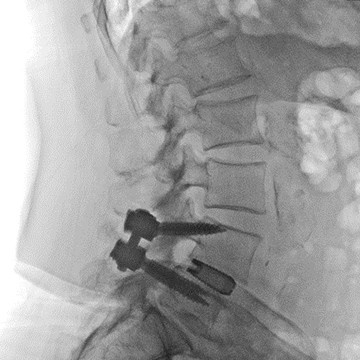
3 Months
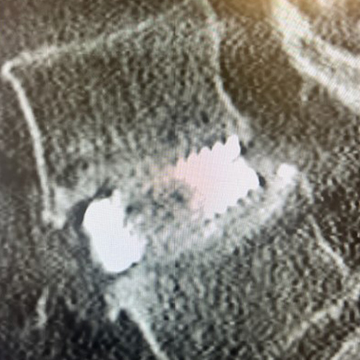
6 Months
Patient #3
24 year old male, with a BMI of 31, presented with cervical spondylitic myelopathy due to large disc herniation at C3-C4, with cord signal changes
—
An anterior cervical fusion (ACDF) with an interbody at C3-C4 was recommended
—
At 3 months, plain radiographs showed full incorporation of the interbody device with a stable fixation at the C3-4 level. There was no evidence of adjacent level junctional disease
—
At 9 months, a CT scan showed evidence of bridging bone throughout the device, although the bridging bone was still incomplete. The patient’s VAS Pain Score had decreased from 7.5/10 to 4/10
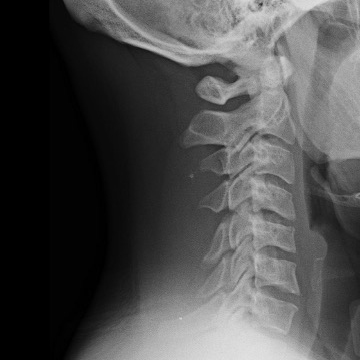
Pre-Op
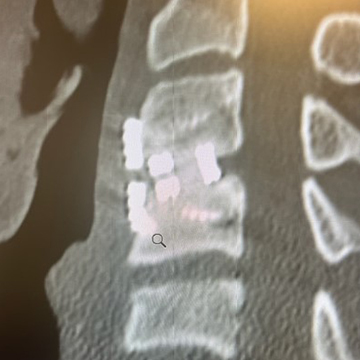
9 Months
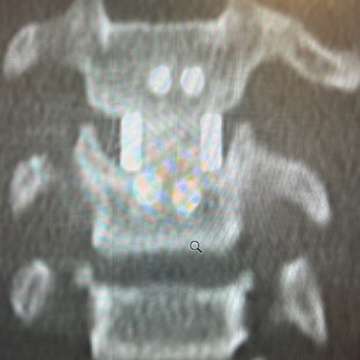
9 Months
Patient #4
A 38 year old male, with a BMI of 22, presented with an L5-S1 left sided disc herniation with leg pain and low back pain in the face of an isthmic spondylothesis at L5. He also had a disc injury at L4-5
—
An instrumented posterior lumbar fusion (PLIF) with an interbody at L5-S1 was recommended
—
At 5 months, the patient reported nearly complete resolution of his back pain and plain radiographs showed an L5-S1 fusion, with a stable construct and no subsidence. His VAS Pain Score had decreased from 6/10 to 1/10
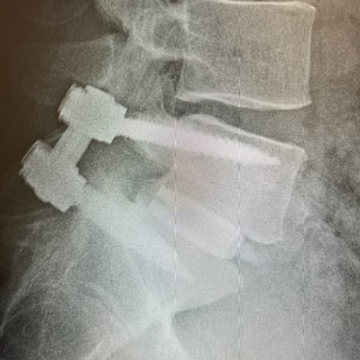
5 Months
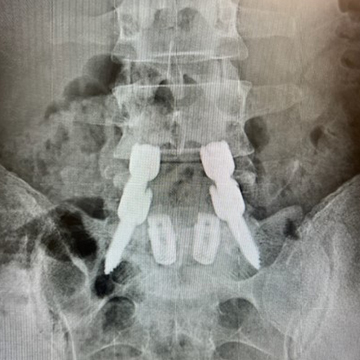
5 Months
2. Kuslich SD, Ulstrom CL, Griffith SL, et al. The Bagby and Kuslich method of lumbar interbody fusion. History, techniques, and 2-year follow-up results of a United States prospective, multicenter trial. Spine (Phila Pa 1976) 1998;23:1267–78
3. Chou YC, Chen DC, Hsieh WA, et al. Efficacy of anterior cervical fusion: comparison of titanium cages, polyetheretherketone (PEEK) cages and autogenous bone grafts. J Clin Neurosci. 2008;15:1240–5. [PubMed] [Google Scholar]
4. Chen Y, Wang X, Lu X, et al. Comparison of titanium and polyetheretherketone (PEEK) cages in the surgical treatment of multilevel cervical spondylotic myelopathy: a prospective, randomized, control study with over 7-year follow-up. Eur Spine J. 2013;22:1539–46.
5. Kurtz SM, Devine JN. PEEK biomaterials in trauma, orthopedic, and spinal implants. Biomaterials. 2007;28:4845–69
